Pregnancy is cause for celebration – when you’re planning for it. Last month, over 100 women from around the country filed a lawsuit against Qualitest Pharmaceuticals, a subsidiary of Malvern, PA-based parent company Endo International, after becoming pregnant due to birth control labeling defects. Philadelphia product liability lawyer Brent Wieand has more on the story.
We expect medications to work the way they’re advertised. We expect Tylenol to soothe headaches, Claritin to ease allergies, insulin to manage diabetes, and birth control to prevent unwanted pregnancies. Unfortunately for 117 Philadelphia-area women, the birth control pills they thought were doing precisely that turned out to be completely ineffective. The pills included brand names:
The catalyst was alarmingly simple: all it took was an accidental 180-degree rotation of the pill-patch markings, which indicate the day of the month. Critically, some of the pills inside a given package of birth control are merely reminder placebos, whose sole function is to help women stick to their daily pill-taking schedule. Therefore, misdated pills aren’t really “birth control” pills at all. It would be roughly equivalent to unknowingly taking a vitamin or a sugar tablet in place of vital medication.
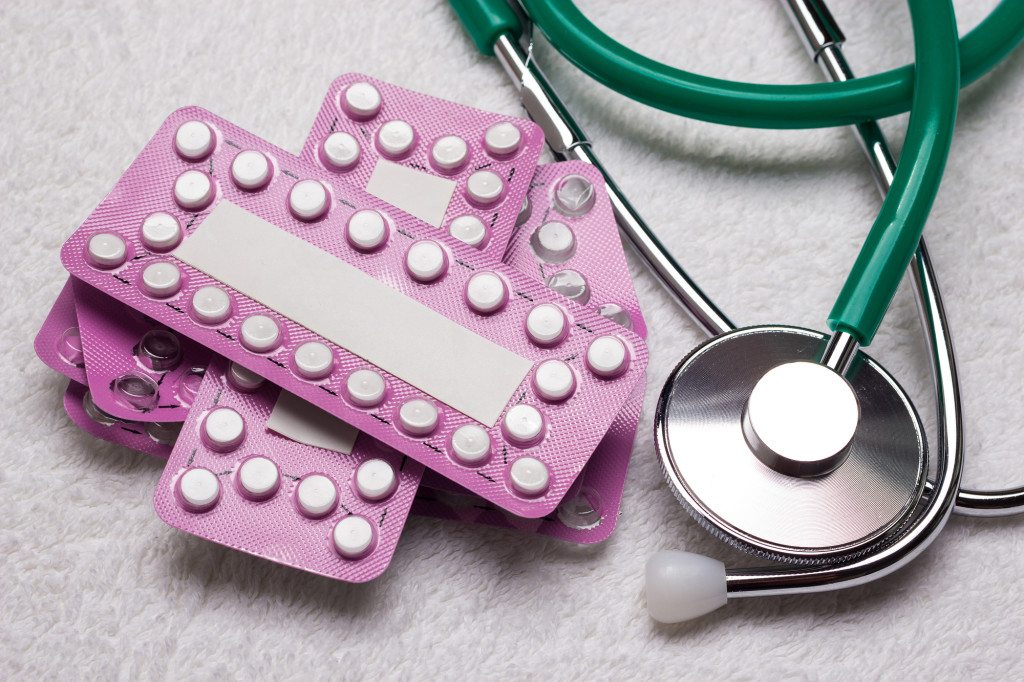
The FDA recalled more than 3 million packages in 2011 after a Missouri woman noticed the rotation and returned the pills to her pharmacy; but for the 117 women who are now suing Qualitest, the FDA’s actions came too late.
Collectively, the plaintiffs are seeking millions of dollars in damages, which they say will be necessary to cover the costs of childcare and education. However, as one Philadelphia attorney cautioned, the plaintiffs may be faced with some legal hurdles, because “under Pennsylvania law, the benefits of having a healthy child are considered to outweigh the detriments of having an unwanted child.”
In addition to Qualitest, whose website says “the company is now ranked in the top ten among all suppliers of generics,” other entities named as defendants include Vintage Pharmaceuticals, LLC –another name for Qualitest, according to Vintage’s profile on LinkedIn – and Patheon [sic], Inc., a pharmaceutical manufacturing company based out of Durham, NC. Qualitest’s parent company, Endo International, is also named in the suit.
This will not be Vintage’s first time meeting with legal controversy. According to FDA News, in 2008 Vintage pleaded guilty to felony charges involving interstate distribution of “adulterated drugs” – about 50 million tablets of the hypothyroidism medication Levothyroxine Sodium, USP – despite “knowing that its methods to assign expiration dates on the drugs did not conform with good manufacturing practices set by the FDA.” In addition to the felony charges for distribution of adulterated drugs, Vintage CEO and President William Propst Sr. pleaded guilty to nearly 20 misdemeanor counts for the same offense, while son William Propst Jr. pleaded guilty to one misdemeanor count. Vintage was fined $4.8 million, and lost over $1 million in profits related to its criminal activity.
After being rejected for class action status by a Georgia federal judge last month, the current case was refiled 800 miles to the north in Pennsylvania, where Qualitest’s parent company is based. Explaining his rationale for the rejection, U.S. District Judge Steve C. Jones stated that each plaintiff’s case should be reviewed individually, because plaintiffs came from different jurisdictions and experienced different outcomes. Among the 117 plaintiffs in the suit, who collectively represent 26 states around the country, 113 actually became pregnant, while 94 carried their babies to term.

Four of the plaintiffs did not become pregnant. Critically, it should be noted that actual harm – not just a close call caused by negligence on the part of the defendant – must be proven in order to win a lawsuit and be awarded damages. As Judge Jones noted, “It is not enough that each class member prove that defendants sold a defective product. Each plaintiff must show in an individualized manner which ‘physical symptoms’ she suffered, her medical history, and whether her use of any allegedly defective product resulted in these physical symptoms or a pregnancy.”
As is often the case in situations where litigation is still pending, Endo International has not released a public statement.
If you or someone you love was injured after taking defective medication that was improperly labeled or manufactured, you may be able to recover compensation to help with your medical bills and other costs. To set up a free, confidential, no-obligation legal consultation, call defective drug attorney Brent Wieand at (888) 789-3161. You will not be charged any fees unless Brent makes a recovery for you.
***Disclaimer: This article is for informational purposes. It is not legal advice and should not be used as legal advice.***